New Drug Designations - December 2023
Shots:
-
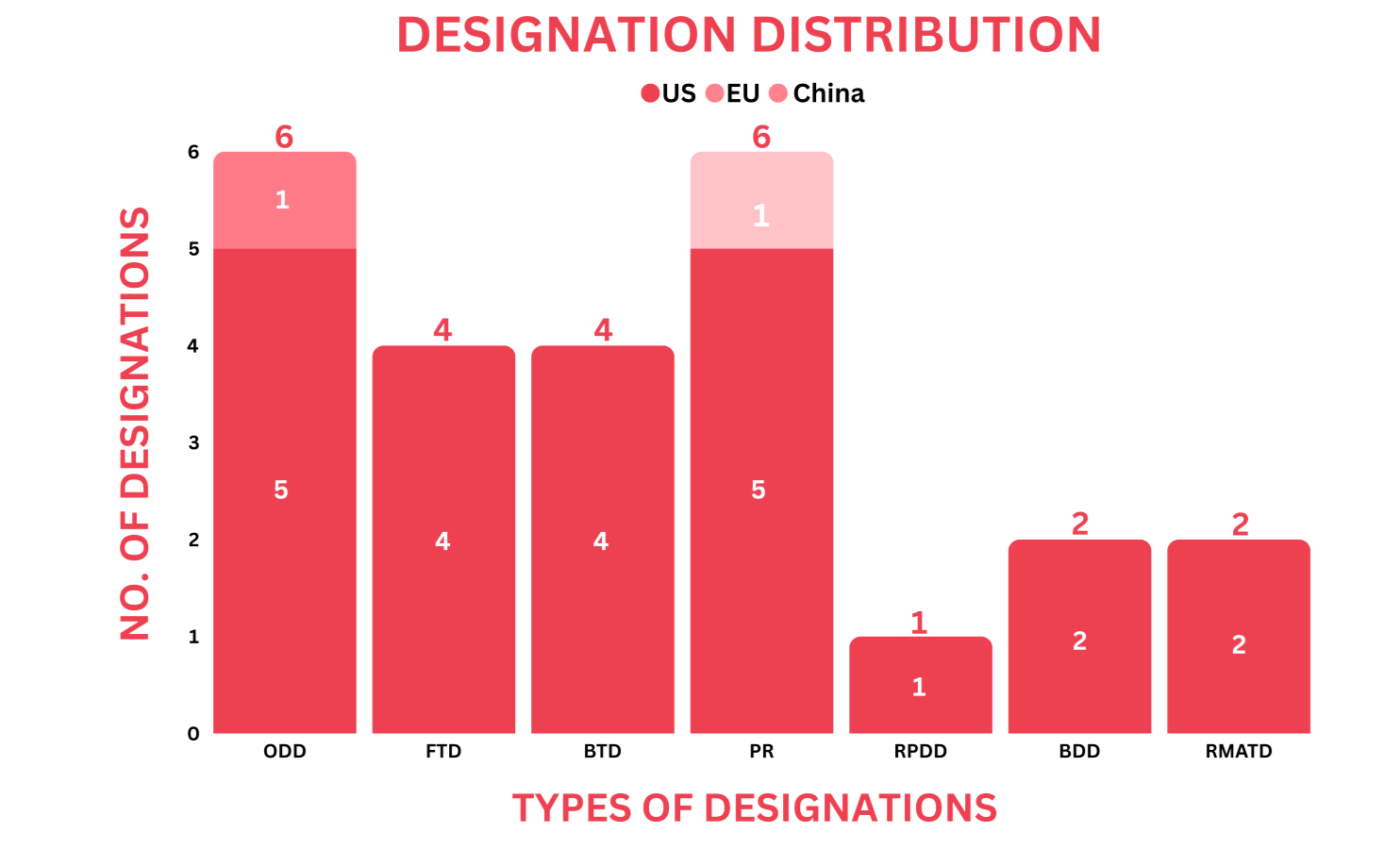
PharmaShots' designation report provides a concise overview of several drugs and their designations by the US FDA, EC, and China’s NMPA. This month’s report includes 6 biological drugs, 10 small molecules, 5 cell and gene therapies, 1 recombinant fusion protein, 1 peptide and 2 devices
-
Neurocrine Biosciences’ Crinecerfont, focused on the treatment of Congenital Adrenal Hyperplasia (CAH), is the drug to receive BTD and from the US FDA along with previous FTD and RPDD
-
PharmaShots has compiled a list of a total of 23 drugs and 2 devices awarded with designations by multiple regulatory bodies in Dec 2023

JR-441
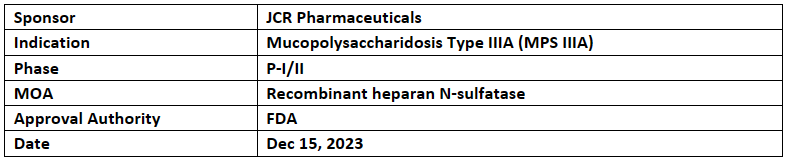
-
JR-441 received ODD from the US FDA, an investigational drug for the treatment of MPS IIIA, or Sanfilippo syndrome type A. In Jan 2022, JR-441 received ODD by the EC
-
JCR is presently conducting a global P-I/II study for JR-441 in MPS IIIA, with the first patient dosed in Oct 2023
-
JR-441 is a form of recombinant heparan N-sulfatase with an ability to penetrate blood-brain barrier (BBB) developed using JCR’s proprietary J-Brain Cargo BBB-penetrating technology
SLS009

-
SLS009 has been given ODD by the US FDA for the treatment of r/r PTCL.
-
SLS009 is being evaluated in an ongoing open-label, single-arm P-Ib/II study in r/r PTCL patients (n=95). This is expected to be registrational study is fully funded by GenFleet Therapeutics and in China.
-
From completed dose-escalation portion of the P-I trial in r/r hematological malignancies. SLS009 showed a favorable safety profile and positive clinical efficacy.
-
AML & Lymphoma patients showed Complete or partial responses, incl. four PTCL patients (36.4%), 1 patient showed complete metabolic response and still ongoing for over 62wks. 2nd patient with CR by CT scan continuing treatment for over 24 wks.
-
Belinostat (HDACi) presently SoC for r/r PTCL in its pivotal P-II showed 25.8% response rate in similar population
-
The P-Ib/II study in PTCL ongoing with top line data expected in 1H' 2024
NS-089/NCNP-02
-
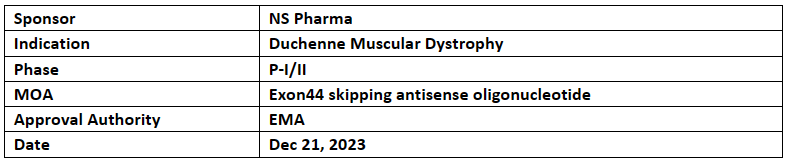
NS Pharma’s NS-089/NCNP-02 received ODD from the European Commission (EC) for the treatment of Duchenne muscular dystrophy on Dec 13, 2023.
-
NS-089/NCNP-02 was discovered through combined research of Nippon Shinyaku & the National Center of Neurology and Psychiatry (NCNP). NS-089/NCNP-02 is an antisense nucleic acid which skips a part of the genetic information of the dystrophin gene and produces a functional dystrophin protein with a slightly shorter chain length.
-
NS-089/NCNP-2 has also received RPDD in Jun 2023 & BTD, ODD in July 2023 from the US FDA
CLS001
-
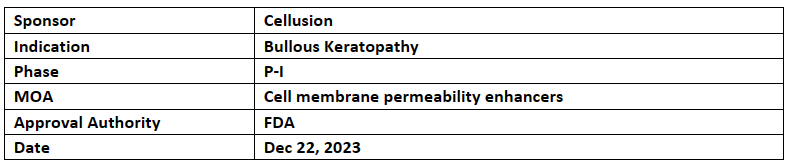
Cellusion’s CLS001 received ODD from the US FDA to treat bullous keratopathy by meeting specific criteria diagnosis, treatment & prevention of rare disease
-
CLS001 is iPS cell-derived corneal endothelial cell substitute and it is alternative cell to corneal endothelial cells
-
Cellusion is preparing for clinical study in Japan as well as for a global study
NXL-004

-
NXL-004, an experimental world’s first AAV gene therapy product being developed by NeuExcell was awarded ODD by the FDA for the treatment of malignant glioma
-
NXL-004 is based on astrocyte-to-neuron (AtN) conversion platform & has shown good efficacy and safety in preclinical trials and in early 2024 will enter the first-in-human study
-
The present SoC of GBM incl. surgery, radiotherapy, and CT results in a mOS of merely 15-18 mos. and a 5-year SR below 10% which creates a huge unmet need for treatment
OCE-205
-
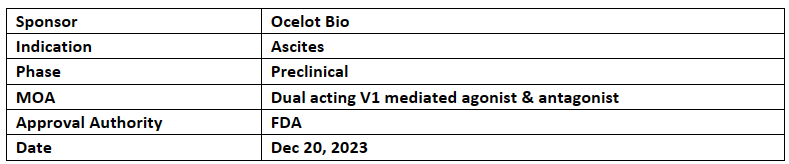
Ocelot Bio’s OCE-205 received ODD from the US FDA for the treatment of Ascites due to all etiologies except cancer in Dec 2023.
-
The company is planning to start OCE-205’s clinical studies in refractory ascites in 2024
-
OCE-205 has also received ODD for hepatorenal syndrome in 2022 and Ocelot has completed the enrolment in a P-II study (NCT05309200) for patients with hepatorenal syndrome with acute kidney injury (HRS-AKI)

Cretostimogene Grenadenorepvec
-
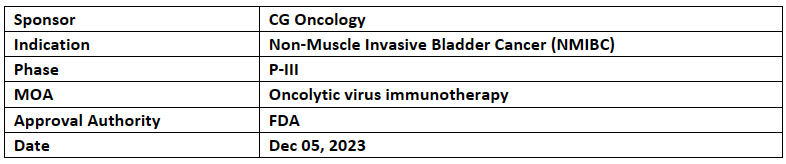
CG oncology’s Cretostimogene Grenadenorepvec received FTD & BTD from US FDA for High-Risk Bacillus Calmette-Guérin (BCG)-Unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) with carcinoma in situ with or without Ta or T1 (papillary) tumors
-
The results from P-III BOND-003 (NCT04452591) study of cretostimogene grenadenorepvec met its 1EP i.e. CRR: 75.7% and was well tolerated. Interim results as of Oct 5, 2023, from 66 patients were presented at SUO’23
-
Cretostimogene grenadenorepvec is being evaluated in an ongoing P-II study (CORE-001) in combination with pembrolizumab for the same indication & also evaluated in an investigator-sponsored study in combination with nivolumab for the treatment of muscle invasive bladder cancer
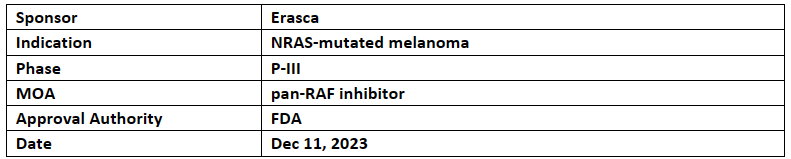
Naporafenib
-
Erasca’s naporafenib in combination with trametinib (Mekinist) received FTD from the US FDA for the treatment of advanced NRAS-mutated unresectable or metastatic melanoma
-
Naporafenib is a P-III ready pan-RAF inhibitor with low response rates & mPFS after treatment & in combination with trametinib shows strong & durable anti-tumor activity
-
Erasca’s P-III study SEACRAFT-2 which will evaluate the clinical efficacy of naporafenib in combination with trametinib (MEKINIST) vs physician’s choice of therapy in NRAS-mutated metastatic melanoma. SEACRAFT-2 will be expected to start in H1'24
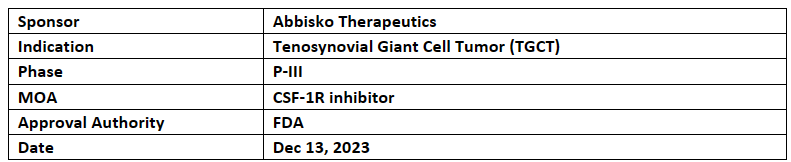
ABSK021
-
AbbiskoABSK021 (CSF-1R inhibitor pimicotinib) received FTD from the US FDA for tenosynovial giant cell tumor ("TGCT"). Previously ABSK021has also received BTD & PRIME designation from the US FDA, China NMPA & EMA for TGCT
-
The P-III of TGCT is a global study conducted simultaneously in the US, China, Europe & Canada. In P-Ib study an ORR of 87.5% in the 50 mg QD cohort of ABSK021 was demonstrated. This data of study was presented at the 2023 CTOS & P-I dose-escalation trial has been completed in the US
-
Abbisko has received an exclusive license from Merck KGaA to commercialize ABSK021 for all indications in Hong Kong, China mainland, Taiwan & Macau. Merck also gave an exclusive global commercialization option, subject to the terms and condition as agreed between both parties
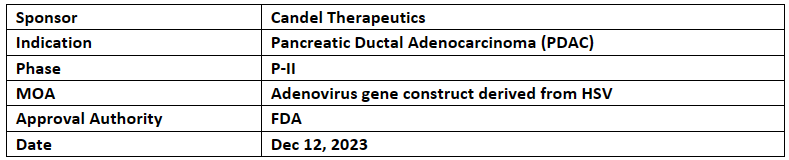
CAN-2409
-
Candel Therapeutics' CAN-2409 plus prodrug (valacyclovir) received FTD from US FDA for Pancreatic ductal adenocarcinoma (PDAC) based on OS results
-
In Nov 2023, Candel presented overall data of randomized, P-II study of CAN-2409 + prodrug (valacyclovir or acyclovir) with SoC in patients with borderline resectable PDAC at the SITC
-
After receiving 2-3 IV doses of CAN-2409, survival rate of 71.4% at both 24 & 36 mos. post dosing in PDAC patients was observed vs 16.7% SoC arm patients.
-
Parallelly, immunological changes observed in the resected pancreatic tissue after CAN-2409 administration suggest activation of effective immunologic antitumoral response in this otherwise “cold” tumor.
-
The P-II study was designed to exclusively focused on borderline resectable disease but after a protocol amendment in 2022, the enrollment of patients with locally advanced PDAC was discontinued but study remains active
-
After P-Ib study completion, no. of CD8+ tumor infiltration lymphocytes TILs increase at the site of the tumor after CAN-2409 treatment
-
The Company’s pivotal P-III study in prostate cancer is being conducted by FDA & Candel will release updated overall survival data in the Q2’24

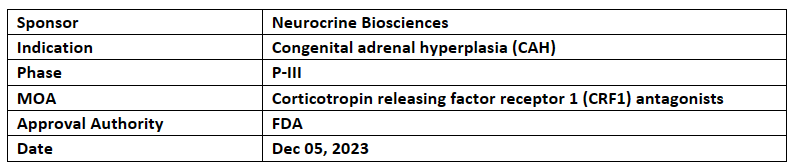
Crinecerfont
-
Neurocrine Biosciences' crinecerfont received BTD designation from the US FDA for congenital adrenal hyperplasia. Crinecerfont has also received FTD & RPDD for the same indication in 2023
-
The safety & efficacy results from the P-III CAHtalyst studies in pediatric & adult patients met 1EPs & 2EPs, showing successful improvement vs SoC in CAH & achieved a >95% completion rate in both studies
-
Neurocrine is also planning to submit NDA in 2024 with USFDA
TAR-200

-
The US FDA has granted BTD to Johnson & Johnson’s TAR-200 for the treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (HR-NMIBC)
-
BTD was given based on results from SunRISe-1 (NCT04640623), an open-label P-IIb clinical study, where participants were randomized to one of in three cohorts to treat with receive TAR-200 in combination with + cetrelimab (Cohort 1), TAR-200 alone (Cohort 2) or cetrelimab alone (Cohort 3) for BCG-unresponsive HR-NMIBC carcinoma in situ CIS patients
-
The 1EPs of the study is Complete Response rate at any time point & 2EPs incl. duration of response DoR, OS, QoL, PK, overall survival, quality of life, PKs, safety & tolerability. Cohorts 1 & 3 were closed to further enrollment effective Jun 1, 2023. Results presented at ESMO’23; AUA’23
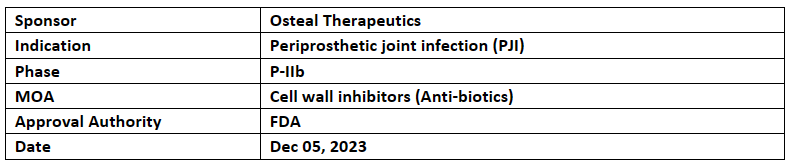
VT-X7
-
Osteal Therapeutic’s VT-X7 received BTD from the US FDA for the treatment of periprosthetic joint infection (PJI) of the hip & knee. VT-X7 has also received OD, FTD, Priority review & QIDP Designation
-
BTD was given based on results from APEX (NCT04662632), a P-IIb, prospective, multi-centre, randomized controlled trial evaluating the safety & efficacy of VT-X7. BTD helps to commercialize the new drug by considering the significant improvement vs SoC
-
Osteal Therapeutic also reports completion of enrolment in APEX-2 the pivotal study
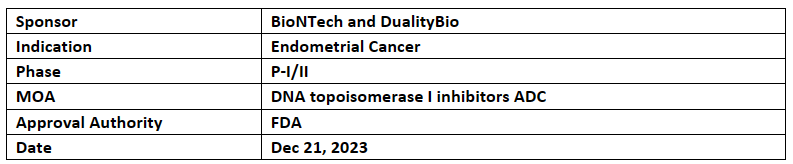
BNT323/DB-1303
-
BNT323/DB-1303 received BTD from the US FDA for the treatment of advanced endometrial cancer in patients who progressed on after treatment with immune checkpoint inhibitors
-
Encouraging results from the ongoing P-I/II study, for which data was also presented at ASCO 2023 and ESGO 2023 demonstrating anti-tumor activity in heavily pretreated patients with advanced endometrial cancer with unconfirmed ORR 58% and DCR 94.1%. Was also found to be well tolerated among advanced solid tumor patients
-
In Jan 2023, BNT323/DB-1303 has already received FTD from the FDA for the treatment of endometrial cancer

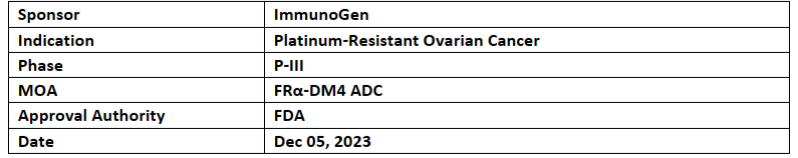
Elahere (mirvetuximab soravtansine-gynx)
-
The US FDA has filed the sBLA based on the P-III (MIRASOL) study of Elahere in platinum-resistant ovarian cancer. The PDUFA assigned date for the application is April 5, 2024, and it has been designated as Priority Review
-
Top-line results were disclosed in May 2023. Elahere’s MIRASOL study depicted statistically significant and clinically improvements in PFS, ORR & OS vs low-grade ocular & gastrointestinal events continue to dominate the safety profile of Elahere
-
In Nov 2022 Elahere was granted accelerated approval by the FDA. For EU and China approved by EMA and NMPA respectively
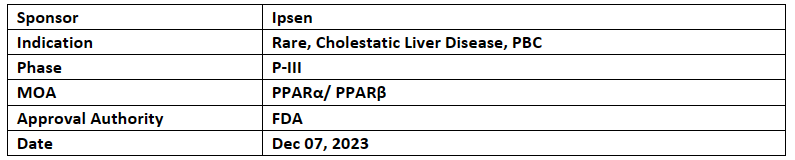
Elafibranor
-
Genfit & Ipsen’s NDA for elafibranor received priority review from the US FDA for the treatment of primary biliary cholangitis (PBC) & the PDUFA date assigned is Jun’24
-
Elafibranor (80mg, QD) is being investigated for its safety and efficacy in the P-III (ELATIVE) study for treating patientswith PBC who are unresponsive or intolerant to ursodeoxycholic acid (UDCA)
-
The EMA has also approved MAA for elafibranor, a CHMP review was submitted on Oct 26, 2023 and UK Medicines & MHRA has been validated the 3rd filing for review
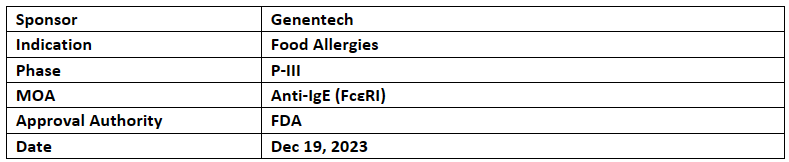
Xolair
-
Xolair (omalizumab) received priority review from theUS FDA for Children & Adults with food allergies. It was based on NIH’s +ve P-III study interim results. Xolair had also received BTD for the severe allergic reactions in Aug 2018
-
The US FDA accepted sBLA based on stage 1 of the NIH-sponsored P-III OUtMATCH (NCT03881696) interim analysis results in patients allergic to peanuts & two other common foods. FDA is expected to make a decision on Xolair in the Q1’24
-
Xolair was first approved in 2003 by US FDA for the moderate to severe persistent allergic asthma. It is also approved for chronic spontaneous urticaria (CSU) & chronic rhinosinusitis with nasal polyps (CRSwNP)
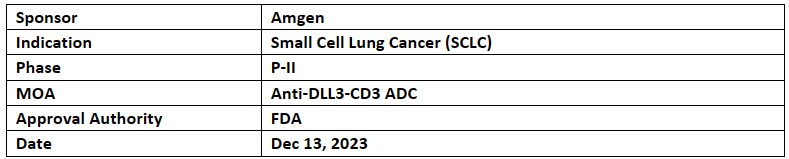
Tarlatamab
-
The US FDA granted priority review to Amgen’s tarlatamab & accepted BLA for advanced small cell lung cancer (SCLC). BLA is based on the P-II study results from the DeLLphi-301 study & PDUFA date for tarlatamab assigned is Jun 12, 2024
-
In the P-I (DeLLphi-300) & P-II (DeLLphi-301) study, tarlatamab showed responses of 23.4% & 40% in patients with advanced SCLC respectively
-
In Oct 23, tarlatamab was granted BTD by the FDA and application is being under the Project Orbis framework and Real Time Oncology Review (RTOR).
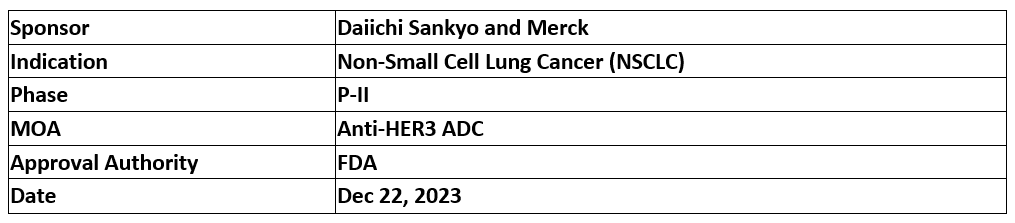
Patritumab Deruxtecan (HER3-DXd)
-
The US FDA has accepted BLA and granted priority review to the Daiichi Sankyo and Merck's Patritumab Deruxtecan (HER3-DXd) for adult patients with locally advanced or metastatic EGFR-mutated NSCLC & assigned PDUFA date for Jun 26, 2024
-
The BLA is based on the P-II (HERTHENA-Lung01) primary study results in which patients (n=225) were randomized 1:1 & received Patritumab Deruxtecan (5.6 mg/kg, IV) once Q3W or an up titration regimen (n=50) received Patritumab Deruxtecan (3.2 → 4.8 → 6.4 mg/kg)
-
The study duration was 18.9 mos., ORR (1EP, 29.8%), mDoR (6.4 mos.), mPFS (11.9 mos.) & safety profile was tolerable, manageable & consistent
Taletrectinib
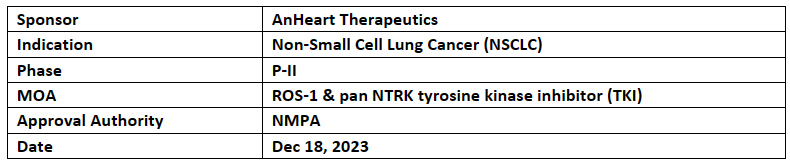
-
AnHeart’s partner Innovent Biologics received PRD from CDE of NMPA for the NDA application for Taletrectinib (ROS1- TKI) used for the treatment of NSCLC. CDE accepted the NDA in Nov 2023
-
The data from the P-II (TRUST-I) study (NCT04395677) is the basis for the NDA and PRD in China was also presented at ELCC 2023. TRUST-II is a 2nd study for taletrectinib at global level (NCT04395677)
-
Taletrectinib was granted BTD by the CDE of China's NMPA in March 2022 and also received BTD by the US FDA

HG302
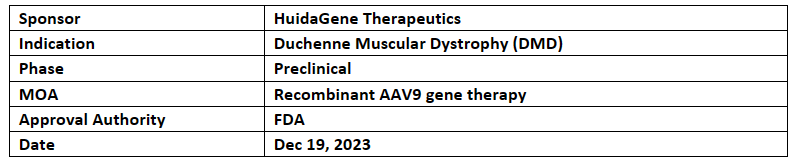
-
HuidaGene Therapeutics' HG302, a novel CRISPR-Cas12 DNA-editing therapy, received RPDD from US FDA for the treatment of Duchenne muscular dystrophy (DMD). This is company’s 3rd program which received RPDD
-
HuidaGene has developed this molecule using proprietary AI HG-PRECISE platform
-
If the HG302 BLA is approved by US FDA for DMD, HuidaGene is expected to be eligible to receive a PRVV

Bladder Care Assay
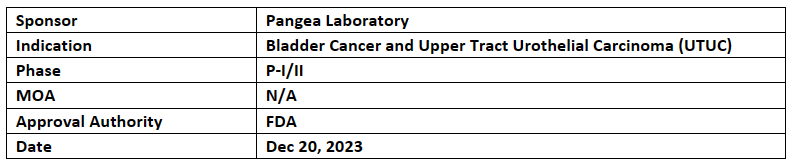
-
Pangea Laboratory’s non-invasive Bladder CARE Assay has received BDD from the US FDA. It is quantitative urine-based diagnosis of bladder cancer or upper tract urothelial carcinoma (UTUC) in patients suffering with hematuria & above-mentioned cancer
-
This assay measures the methylation levels of three biomarkers specific to UCC from single qPCR. The analysis outperformed traditional cytology and other FDA-approved tests, with specificities of 93.5% & 92.6%, 96.0% & 88.0% and 89.0% for the detection of bladder cancer, UTUC and sensitivity for carcinoma in-situ respectively
-
Pangea laboratory is planning to start a multi-center trial to gain approval for Bladder CARE Assay
RadioGel Precision Radionuclide Therapy 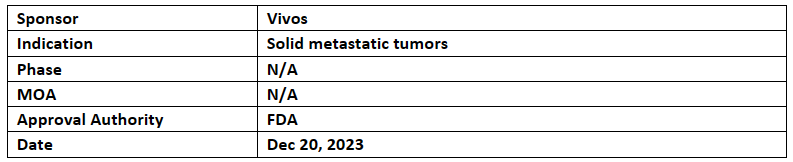
-
Vivos’ RadioGel precision radionuclide therapy received BTD from the US FDA
-
Animal study data verified Radiogel’s effectiveness & safety. Vivos & mayo clinic currently working together for use of RadioGel delivering therapeutic radiation to solid metastatic tumors associated with other indication
-
RadioGel containsyttrium-90 phosphate microparticles which can directly administer into a tumor & localizes the dose within the treatment area by more than 90% of short-range beta radiation within 10 days so that tissues & normal organs are not adversely affected

OCU400

-
Ocugen’s OCU400 received RMAT designation for retinitis pigmentosa (RP) associated with RHO mutations. It is based on preliminary clinical data of OCU400 -101 P-I/II study as measured by LLVA, MLMT & BCVA
-
In the P-I/II study of OCU400, 86% (6/7) patients experienced either improvement or stabilization in MLMT scores from baseline, among which 29% (2/7) experienced 3 Lux luminance level improvement
-
The data of gene-agnostic MOA of OCU400 suggest that it may be able to treat a group of RP & LCA patients. Ocugen’s intends to submit the safety & efficacy data to the FDA to expand its RMATD & for marketing authorization application and it is working with the FDA to decide the protocols necessary to advance the P-III study
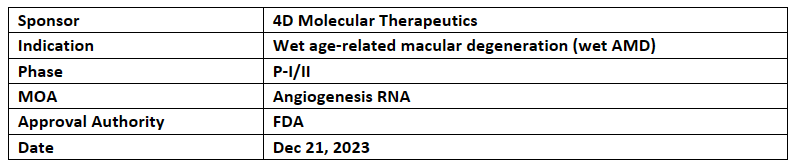
4D-150
-
4DMT’s 4D-150 received RMATD from the US FDA for Intravitreal treatment of wet AMD. . RMAT designation follows PRIME designation received from the EMA in Oct 2023
-
This RMATD is based on the interim results of P-I PRISM study which demonstrated tolerability, safety & clinical activity profile of 4D-150
-
4DMT is working on preliminary P-III study plan with the EMA & the US FDA & an update will be expected in Feb 2024 with interim randomized P-II PRISM clinical data from advanced, high treatment need wet AMD patients
Related Post: New Drug Designations - November 2023
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.